Nuclear Radiation
Nuclear Radiation
Radioactive decay is the emission of particles or electromagnetic radiation from radioactive elements. The emitted radiation can be both useful and dangerous.
Radioactivity
Some nuclei are unstable and they release radiation as they change in order to become more stable. This is a process called radioactive decay.
Radioactive decay is a random process. This means that you can’t predict when a single nucleus will decay.
Instead, we can measure the activity of a radioactive source. This is the rate at which the source of unstable nuclei decays and it is measured in becquerels \text{(Bq)}. 1\text{ Bq} is equivalent to 1 \text{ decay per second}.
The count rate is the number of decays recorded per second by a radiation detector such as a Geiger-Muller tube.
There are three main types of radiation you need to know. The table below summarises their properties:
| Type of Radiation | Symbol | What is it? | How ionising is it? | Absorbed By |
| Alpha (\alpha) | ^4_2He\text{ or }^4_2\alpha | 2 protons and 2 neutrons (a helium nucleus) | Strongly ionising | A few \text{mm} paper |
| Beta (\beta) | ^0_{-1}e\text{ or }^0_{-1}\beta | High energy electrons | Moderately ionising | A few \text{mm} aluminium |
| Gamma(\gamma) | \gamma | Electromagnetic radiation | Weakly ionising | Many \text{cm} lead |
Nuclear Equations
Nuclear equations are used to represent nuclear decay.
The emission of alpha or beta radiation causes a change in the nucleus. You need to be able to work out the mass and atomic numbers of nuclei after radioactive decay by balancing the mass numbers and atomic numbers on both sides of nuclear equation.
Alpha Decay
Here is an example of alpha decay:
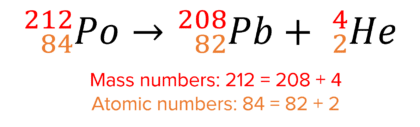
In alpha decay, the mass number decreases by 4 and the atomic number decreases by 2.
Beta Decay
Here is an example of beta decay:
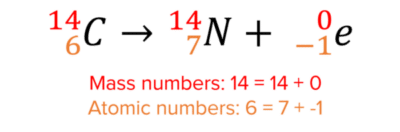
There is no change in the mass number of the nucleus since beta-particles contain no neutrons. Beta-particles contain 1 electron however, and so to balance the atomic numbers on both sides of the equation, the atomic number of the nucleus must increase by 1. This turns the nucleus into a nitrogen nucleus.
Gamma Decay
Here is an example of gamma decay:
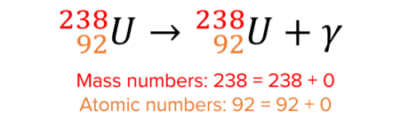
Gamma decay does not change the mass or atomic number because gamma particles have no mass.
Nuclear Radiation Example Questions
Question 1: What unit is used to measure activity?
[1 mark]
Becquerel \text{(Bq)}.
Question 2: Put the three types of radiation in order from shortest to longest range.
[2 marks]
alpha, beta, gamma.
Question 3: Match the types of radiation to the parts that they contain. Some radiation types may contain more than one part.

[4 marks]
One mark per correct line drawn.
Question 4: Calculate the missing value in this nuclear equation:
![]()
[1 mark]
So the missing value is \bold{171}.
Question 5: A ^{137}_{56}\text{Ba} nucleus undergoes radioactive decay. The nucleus after decay is still ^{137}_{56}\text{Ba}. What type of radiation was emitted?
[1 mark]
Gamma radiation.
Because there is no change in the mass or atomic number.
Specification Points Covered
AQA GCSE
- 4.4.2.1 Radioactive decay and nuclear radiation
- 4.4.2.2 Nuclear equations